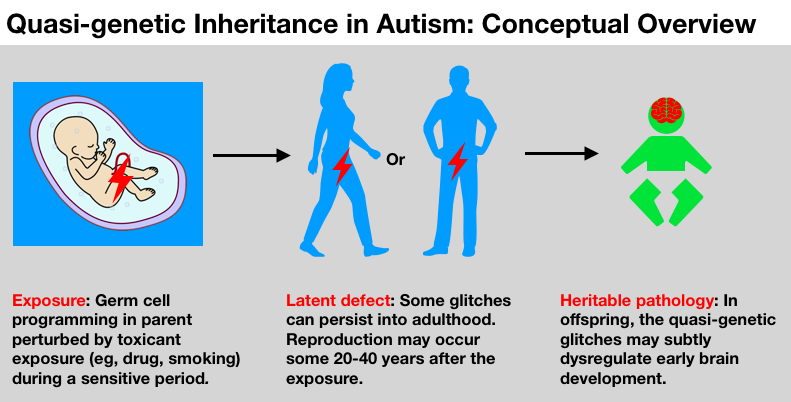
Though it’s impossible to have a genetic epidemic in the classic sense, certain exposures to reproductive cells may quietly engender a quasi-genetic epidemic. The authors explain the science and suggest a new priority for autism research.
By La Donna Ford, MD, and Jill Escher
Answers regarding the causes of autism have been frustratingly elusive. Only a fraction of cases have been attributed to genetic mutations, and even fewer to fetal exposures. Despite decades of intensive research, the vast majority of autism cases remain head-scratchingly idiopathic, that is, without a known cause.
So what hidden forces could be fueling the dramatic increase in prevalence, which has reached an alarming 1 in 59 children? And how could a disorder seen to be strongly heritable increase, at all?
In this article we explain the “quasi-genetic” hypothesis of autism, a relatively new concept that could help answer those questions. It could help explain autism’s heightened risk among siblings even in the absence of genetic causes (often called the “missing heritability” of autism), its swift increase in prevalence over the past three decades, even its skewed sex ratio, among other puzzles. You will see that while it’s impossible to have a “genetic epidemic” in the classic sense, it is indeed possible to suffer a quasi-genetic epidemic, given the right conditions.
“Glitches in these molecules can alter the way genes are expressed without changing the underlying genetic code.”
What do we mean by this quasi-genetic stuff? Aren’t all of our inborn traits genetic? Yes, we would say most are. But our egg and sperm (also called germ cells), highly specialized cells that are exquisitely and minutely programmed to enable the development of an entire human, are not merely sacks of DNA. They also harbor billions of molecules that help control how and when genes function — poised to turn them up or down, depending on the time and the tissue. Glitches in these molecules can alter the way genes are expressed without changing the underlying DNA code. In this way, even without a mutation, non-genetic glitches can derail normal development.
We realize this realm of science is unfamiliar to most folks. Certainly it appeared nowhere in our high school biology textbooks. But over the past decade or so it has become increasingly clear that these quasi-genetic factors help modulate development, and, moreover, that they can be vulnerable to a host of environmental factors.
Egg (left) and sperm (right, as spermatogonia, the progenitors of sperm) contain the complex multi-layered molecular code for building a new human. Their heritable content is multi-layered and not limited to the DNA sequence. (Images from Embryology Education and Research website embryology.med.unsw.edu.au/embryology)
The quasi-genetic elements include the “epigenome” and “chromatin” of our cells. The epigenome essentially refers to the network of chemical compounds attaching to and surrounding DNA that modify genetic function. Chromatin basically refers to the DNA and its structural packaging which condenses and expands DNA in a way that helps regulate gene expression. These basic elements are fundamental to the differentiation and functioning of all our cells: while every cell features the same genome, these quasi-genetic forces help determine whether a cell becomes a brain cell or a skin cell, for example, and how those cells behave.
When toxicants cause glitches in the quasi-genetic machinery of our germ cells, the effects are seldom straightforward, and they may or may not disturb the development of offspring. The glitches could be repaired down the line, could be negligible, or could be considerable. They might differ according to underlying genomic difference, or a multitude of complex biochemical processes shifting over time. The same exposure can exert different effects in different stages in different people. But in the end, as we will discuss, research has linked exposure —> germ cell glitches —> offspring perturbation of brain/behavior owing to quasi-genetic mechanisms. In biology, germ cells are increasingly appreciated not just as storage vaults for DNA, but as dynamic biological entities that can be responsive and vulnerable to environmental cues. [Alert to biology buffs: we are not discussing “transgenerational” effects, those effects that persist in the absence of a direct germline exposure. Rather, for the purposes of this article we are solely concerned with direct germ cell exposure.]
Now it’s time for us to dive deeper into science and history to explain why, despite some we’ve-turned-over-every-stone malaise in the “genes” or “environment” world of autism research, there is an entire dimension of risk that has yet to be pursued. And we can’t think of a better place to start our journey than in anesthesia research lab in New York City, circa 1980.
Turndorf was concerned about how common volatile anesthetic gases affected brain function in exposed fetuses, and also the next generation.
Dr. Turndorf’s anesthesia-exposed mouse eggs
In the 1950s and 60s America experienced a chemical revolution — our fields clouded with new pesticides like DDT, clothing glowed with vibrant synthetic dyes, kitchens filled with Tupperware, and pharmacies brimmed with new synthetic drugs. In the field of anesthesia, brilliant chemists synthesized novel volatile anesthetic gases that helped keep patients sedated and inert during surgical procedures and were safer to use than their predecessors. Though utterly miraculous in enabling modern surgery, these powerful chemicals also had their downsides, among them possible neurological damage.
In about 1980, Herman Turndorf, MD, Professor and Chairman of Anesthesiology at the New York University School of Medicine, and colleagues wanted to see what effect commonly used general anesthetic agents (“GA”) had on the learning and behavior of mice exposed in the womb. Translated to a real-world question, they sought to discover whether surgical anesthesia in a pregnant woman might cause brain impairment in her exposed child. Anesthesiologists like me (LDF) would of course want to know the long-term impacts of our sedation techniques, as would our patients.
In a succinctly written 1981 paper, the team reported that the baby mice borne of gestating females exposed to two GA agents called halothane and enflurane suffered long-term learning impairment, performing poorly in food maze tests compared to control mice. This was not entirely unexpected. But, to our eyes, an additional finding stands out.
The researchers did something remarkable in that they also looked at learning outcomes in six mice in the next generation. These mice were the grandpups of two females exposed to halothane as fetuses. These grandpups were just early-stage eggs, nested inside fetal ovaries of their fetal mothers, at the time of exposure.
An exposure to a pregnant woman can affect three generations: the mother, her fetus, and her fetus’s nascent germ cells.
Turndorf and colleagues seemed to understand that an exposure to a pregnant female can simultaneously affect three generations: the mother, her fetus, and her fetus’s germ cells. Because those germ cells are the delicate precursors to the gametes containing the fundamental blueprint for the next generation, contamination by a chemical or drug could therefore impair the grandchildren, born decades after the germ cell exposure.
“The grandpups’ impaired learning suggested the anesthetic agent may have caused a ‘genetic aberration’ in the exposed mothers’ fetal eggs.”
In their report, the researchers remarked on the outcomes of the grandpups borne of the exposed germ cells. It turns out they were found to be “significantly slower than control mice throughout the training” on all days of testing and all configurations of the maze. The researchers concluded that the grandpups’ impaired learning “suggests that the anesthetic agent may have caused a genetic aberration” in the exposed mothers’ fetal eggs (Chalon et al., 1981).
After this 1981 paper, Turndorf’s lab returned to this question of germ cell effects of general anesthesia in a different type of mouse experiment. Knowing that the GA agent enflurane caused damage to sperm (citing Land et al., 1981) and that halothane caused learning impairments in the generation borne of exposed eggs based on their own observations, the lab investigated the possibility that exposure of adult male mice to enflurane prior to mating could also affect the brain function of offspring, due to damage to the exposed sperm.
Once again, they found impaired learning function in the generation borne of the exposed germ cells, this time later-stage sperm instead of early-stage eggs. They remarked that it “seems likely that spermatogenetic changes, caused by enflurane, are associated with genetic alterations” that affected the pups’ brain development (Tang et al., 1984).
Mental pathology rooted in germ cell exposure: a scientific heresy?
Therefore, we can see that in the early 1980s two papers suggested adverse heritable effects of GA, showing mental impairment in the progeny via mysterious “genetic aberrations” or “genetic alterations” of female or male germ cells.
Now, one might think these findings would have raised some concerns in the medical community — surely, if GA agents could damage our sperm and eggs’ genetic material in a way that caused learning deficits in the next generation, that is something doctors and patients would wish to know. But instead what followed was the opposite of scientific scrutiny — decades of absolute silence with absolutely no follow-up research that we could find.
Though it’s difficult to say why the germ cell exposure idea hit a wall, it seems possible that the observations reported by Turndorf’s lab fell victim to the weight of conventional dogma about inheritance. It was broadly accepted at that time that heritability of traits depended on genes from our parents, except in those rare cases where genes suffered a random mutation. The dogma left no room for other ideas about molecular sources of inheritance, such as the quasi-genetic forces we discuss here, forces that did not rise to the level of a full genetic mutation. The GA agents were not thought to be mutation-causing, so the idea that GA could induce a heritable brain pathology amounted to a sort of scientific heresy. The idea, it appears, became scientifically orphaned. Abandoned.
Looking back on my years in medical training and anesthesiology practice, I (LDF) should note that not once did the question of heritable effects of germ cell exposure to GA come up. Germ cell effects were not mentioned in medical school, residency, FDA advisories, research papers, conferences, or professional literature. It was as if germ cells were almost presumed to be immutable marbles, impervious to even the most toxic chemical influences like GA.
In a Florida lab, “epigenetic inheritance” links germ cell exposure to mental impairment
Now, fast forward from 1980s New York City to a few years ago in Gainesville, Florida. In the early 21st century, cracks in the edifice of genetic determinism began to appear, and these became known to Dr. Anatoly Martynyuk, Professor of Anesthesiology and Neuroscience at the University of Florida, who researches developmental neurobehavioral impacts of general anesthetic agents. The professor, while not familiar with the Turndorf studies, had read a series of animal studies demonstrating that acute stress and trauma could impact the molecular content of egg and sperm, resulting in altered brain and behaviors in the offspring, one of the new lines of research demonstrating mechanisms of non-genetic inheritance (see, eg, Bohacek and Mansuy, 2015). So he started thinking beyond the GA-exposed brain to consider the exposed germ cells as well.
Perhaps, he thought, GA could be meddling with molecules inside nascent eggs or sperm.
The previous decade of research demonstrated that common GA agents such as halothane, enflurane, and sevoflurane could not only influence neuronal function, but also induce epigenetic and chromatin modifications, though this work was not done in germ cells in particular (Csoka et al., 2009; Pan et al., 2006; Rampil et al., 2006; Jia et al., 2016; Vutskits et al., 2018). Again, these changes include molecular alterations to the DNA three-dimensional structure and chemical tagging of DNA, perturbing the way genes are expressed. For example, even brief exposure to the GA agent isoflurane led to widespread changes in genetic control in a brain region called the amygdala six hours after exposure (Pan et al., 2006).
So, hypothesizing that GA exposure to early germ cells could cause a direct “epigenetic inheritance” by changing how germline genes function, Martynyuk’s group undertook an experiment. They exposed both male and female neonate rat pups to sevoflurane, the most popular GA gas used in pediatrics, and then looked at brain, gene expression, and behavior in the next generation, taking care to assess sex-specific effects, since sperm and egg are epigenetically distinct. Remember learning about meiosis in high school? Egg and sperm travel very different developmental paths, and that includes the content of their epigenomes.
The lab used a sub-clinical dose of sevoflurane because a clinically relevant dose would have resulted in low oxygen levels and other abnormalities in the pups’ blood (use of GA generally requires use of a breathing apparatus to keep the patient alive, something the researchers could not do in this case). With limited funding they only looked at only two parts of the brain (hypothalamus and hippocampus) and the expression of only two genes. But it was a start, and as far as they knew, it was the first study to examine the heritable impacts of GA.
After assessing the effects on the directly exposed pups (which predictably suffered some impairments) the team looked at brain, gene expression and behavior outcomes in the following generation, which we’ll call “progeny,” borne of the pups’ GA-exposed germ cells.
They found that the male, but not female, progeny showed signs of neurodevelopmental impairment. Progeny of exposed males, that is, of the exposed sperm, had abnormalities in the maze test, suggesting impaired cognition, abnormalities in prepulse inhibition of startle, suggesting decreased ability to filter out unnecessary information, and decreased expression of a gene in the hypothalamus. Where both parents were exposed, male progeny exhibited impaired spatial memory and decreased expression of the gene in both the hypothalamus and hippocampus. An analysis of epigenetic changes in sperm of exposed males and brains of progeny revealed gene expression shifts not present in control rats. In other words, it appeared that the male rat progeny, exposed only during the early germ cell stage, exhibited behavioral impairments connected to sevoflurane-induced epigenetic modification (Ju et al., 2018).
It was not a stop-the-presses sort of study, given the limited scope of investigation, the sub-clinical doses of the drug, and the subtleties and oddities of some of the findings. But it was nevertheless suggestive that GA seemed to induce a non-genetic effect in early-stage germ cells, causing some sex-specific brain and behavioral abnormality in the next generation. And this time, it seems, the lesson was not entirely lost on the medical community.
A British Journal of Anaesthesia editorial accompanying Martynyuk’s paper, and also citing the first Turndorf study, touched on the possible public health implications of the new findings. The commentary, evocatively titled, “A poisoned chalice: the heritage of parental anaesthesia exposure,” noted that “we are faced with a real possibility that general anaesthetics are not innocuous agents that ‘only put children to sleep’ but rather formidable modulators of chromatin remodeling and function” perhaps modulating developmental neuroplasticity in the next generation (Vutskits et al., 2018).
The importance of “critical windows” in germ cell exposures
Now, you are probably thinking, and you would be correct, that this is all very interesting but GA exposure to germ cells can’t generally cause autism, because otherwise nearly all kids would have autism. After all, a great many parents have had general anesthesia at some point in their pre-conception lives, whether for a tonsillectomy, appendectomy, something dramatic like major heart repair, or perhaps birth under sedation for a C-section. And clearly, although autism has increased markedly in prevalence, it’s still limited to about 1-2% of the childhood population. Common sense suggests gametes must be largely protected from damage.
But we are not suggesting such widespread havoc at all. Instead we should think about what scientists call “critical windows,” and also dosages. You see, it’s not just the substance itself, but the timing and the dose that make the poison. You may remember, for example, the story of a sedative drug called Thalidomide which came into use in the late 1950s. That drug often caused horrific birth defects such as missing limbs — but only if consumed in a certain early weeks of embryonic development. After that phase, this acutely toxic drug had fairly benign effects.
“It’s not just the substance itself, but the timing and the dose that make the poison.”
A similar timing-matters phenomenon exists with germ cells. Now bear with us because here we must delve into more molecular biology. Early in their careers, during the fetal period and, for girls, also the infancy stage, human germline DNA undergoes a dynamic de-nuding and redecorating unlike any sequence of events in other cells.
The germ cells, in order to give rise to an entire new organism, need to shed old epigenetic rags and dress themselves with new epigenetic finery consistent with their sex, male (sperm) or female (egg). Broadly called germline reprogramming, this process sees the germ cells’ DNA become “demethylated,” “remethylated,” and “imprinted” in sex-specific ways. At the same time it appears that the chromatin and the protein spools that wrap DNA, called histones, also get remodeled. Together with other mechanisms, these are the marks (or absence thereof) that will fine-tune development. Our germ cells are “immortal” — descending from millions of years of organismal continuity — because of this elaborate molecular ritual that imbues them with new youth and totipotent superpowers.
You could imagine these molecular processes a bit like a game of musical chairs. The young denuded DNA extends like the line of empty chairs. The chemical tags that attach to and fold the DNA swarm like the kids running around looking for an open seat. DNA function will ultimately change depending on what chairs end up occupied by what kids, and how much those chairs get pushed around.
Oversimplistic, absolutely, but it gives you a sense of the dynamism present in the early germ cell, which contrasts with later developmental stages in which germ cells tend to be somewhat sleepier. Once the kids find their seats, the music stops and they sort of hang out, for years. Male germ cells, however, feature some notable additional epigenetic and genetic vulnerabilities from puberty onward due to the vagaries of spermatogenesis. Picture the girls lounging in their seats, but the boys pushing more chairs around as they hit puberty. Therefore, for men in particular, pubertal or later exposures (for example, drugs or tobacco) could damage the molecular program in their germ cells.
“A potent, epigenetically active drug like GA, delivered in the right time, duration and dose, could interfere with how the genome gets folded and decorated. Some of those quasi-mutations can persist post-conception, into the offspring born years or decades later.”
Enough biology — we know this is difficult, thank you for bearing with us — and back to the bottom line. A potent, epigenetically active drug like GA, delivered in the right time, duration and dose, could interfere with how the germ cell genome gets folded and decorated. Some of those glitches can persist post-conception, into the offspring born years or decades later, exerting outsize, and rather unexpected, effects on gene expression. Thus, we can have a quasi-genetic impact on health and development.
Autism family stories raise red flags
Okay, so certain exposures like general anesthesia might tinker with our germ cells. But what does it mean for autism? A handful of animal studies finding neurodevelopmental impairment hardly amounts to a closed case. Well, regrettably there appear to be no published papers on heritable effects of GA in humans, which strikes us as a galactic, scream-worthy gap in the research, considering the magnitude of synthetic volatile GA use since the 1950s. So of course it’s impossible to draw any conclusions.
But perhaps it’s useful to begin where human research often starts, by simply listening to families and hearing their stories. Might their reports raise some red flags? While this is hardly science, we wanted to share a sampling of autism family stories that seem to do just that.
A mother reports her mother had an appendectomy while pregnant with her. She has two girls with idiopathic autism. A mother said her mother had surgery when pregnant with her, following an automobile accident. She has three boys with idiopathic autism. Two different fathers report having had a series of complicated surgeries after suffering teenage gunshot wounds. Both have sons with profound forms of idiopathic autism. Two different parents, one female, one male, had open heart surgery in early childhood to repair heart defects. Both have sons with idiopathic autism. A mother states she had two surgeries as a neonate, one to remove a benign tumor and another to repair a hernia. She has two sons with idiopathic autism. A father’s mother said she had surgery when pregnant with him, to correct a placental problem. The father has a son with idiopathic autism. A mother had early childhood surgeries to repair a cleft palate, She has a son with idiopathic autism. Now, these are mere anecdotes without control groups. But from evidence when available, as a makeshift control group where the autism parents’ siblings were not exposed to early surgery (and therefore GA) there were no other family cases of autism.
Still, as we said, this is at best hypothesis hunting and certainly not science. And who knows what if any genetic predisposition or other exposure questions may have lurked in the parents as well. But our point in sharing the family stories is not to convince you that parental germ cell exposures to GA can raise the risk of autism in progeny — as we said, there has so far been zero research in humans. Instead, we are suggesting these stories, reflecting strong patterns of heritability, combine with lab science and textbook knowledge on GA impacts to present an exquisitely important question for research: are some cases of “heritable” neurodevelopmental pathology not genetic at all, but rather quasi-genetic and induced by long-forgotten germ cell exposures? We can only find out if we first ask the question.
As a former anesthesiologist (LDF), let’s be clear. This is not about blame or antagonism toward necessary drugs or especially GA, which is by all accounts one of the great medical advances of the modern age. If you want to get a sense of the surgical horrors endured by humans before the advent of GA, we invite you to watch this documentary, Scream: The History of Anaesthetics. A great many readers of this article are alive today thanks to the miracle of modern GA, whose sedative and hypnotic effects enabled the practice of modern surgery. Our gratitude for GA should overflow, but at the same time we should be cognizant that these agents are powerful poisons that may invite unintended consequences.
So far we have used GA as an example toxicant, but when it comes to contemplating the reality of families’ biological histories, a great many other exposures should also concern us.
Drugs aplenty in the post-war womb
Synthetic drugs, including those used in pregnancy, boomed during the postwar decades. In addition, maternal smoking peaked in the 1960s. Some examples of common pregnancy drugs from the 1950s and 60s, pictured clockwise from top left: anti-nausea, anti-anxiety, synthetic steroid hormones [pictured here, an ad for the notoriously toxic drug DES], volatile anesthetic gases, tobacco, barbiturates, amphetamines/methamphetamines.
Why now? Why the steep increase in autism starting with births in the early 1980s? It’s a baffling mystery seemingly without any explanation. So a song springs to mind, “Don’t know much about history… don’t know much biology...” because it seems if we don’t know much about our biological histories, we may never piece this puzzle together.
There’s no nice way to put it: the American womb became something of a chemical soup in the decades after World War II. As writer Annie Murphy Paul observed, the post-war years saw a staggering increase in the use of synthetic pregnancy drugs. “The middle of the twentieth century was a golden age of pharmaceutical innovation, a time when serene sleep and steady nerves and a slim figure could be found inside the medicine cabinet,” she writes. “Pregnant women, too, were promised relief from all the complaints, small and large, of their condition: sleeplessness, morning sickness, miscarriage… those who gave birth in the postwar years, writes one chronicler of the period, ‘were among the most medicated women in history’” (Paul, 2010).
Pregnancies of the post-war era were often heavily medicated with chemical compounds that were entirely unprecedented in the human womb. Here, for example, a medical record from 1965 shows that a pregnant woman in Boston was prescribed synthetic steroid hormones, diuretics, anti-nausea drugs, analgesics, and sedatives, among other drugs such as insulin for Type 1 diabetes. While any quasi-genetic impact is of course unknown, it is worth noting that three of the woman’s grandchildren exhibit multiple idiopathic neurodevelopmental abnormalities.
Synthetic hormones, barbiturates, amphetamines, diuretics, analgesics, sedatives, anti-anxiety medicines, tobacco. All of these were rampantly used in pregnancy, typically under doctors’ orders. It was not uncommon for pregnant women of the 1950s and 60s and even the 70s to take upwards of a dozen prescription and over-the-counter drugs for common or serious complaints. The placenta was presumed to provide a barrier to harm, pregnancy drugs were seldom evaluated for safety or efficacy, and women at that time tended to trust without question the advice of their physicians.
[On a personal note, back in the 1960s co-author JE was exposed in utero to an intensive eight-month protocol of synthetic steroid hormone drugs, a history detailed in Bugs in the Program (Escher, 2018), while co-author LDF was exposed in utero to tobacco smoking.]
Meanwhile, of course neither regulators nor physicians considered potential impacts of all these drugs on the exposed fetus’ germ cells. It amounted to a vast uncontrolled chemical experiment, with wholly unknown generational implications. But today the tide is turning, and finally researchers are beginning to examine links between drugs, germline disruptions, and impairments in offspring, including impacts on brain and behavior. Beyond the previously discussed general anesthetic agents, here are some examples from the research literature:
Steroid hormones
Steroids are little molecules that help orchestrate development by changing gene expression. Synthetic, lab-made steroid hormone drugs, including gender-bendy fake sex steroids, came into widespread use in pregnancy in the 1950s and 60s. Many people will remember, for example, the toxic synthetic estrogen, diethylstilbestrol (DES) one of the greatest medical disasters in history. While this drug was taken by millions of pregnant women for the ostensible prevention of miscarriage, it was in fact ineffective and often carcinogenic (causing reproductive cancers) and teratogenic (causing birth defects such as penile and uterine malformations). In repeated mammal and human studies, DES has been linked with grandchild pathologies such as cancer and reproductive dysfunction, suggesting germ cells were tainted by the pseudo-hormone’s disruption of normal cell signaling (see, eg, Titus et al., 2019). And notably, last year significantly elevated odds for attention deficit hyperactivity disorder (ADHD) were found in grandchildren of women who took DES during pregnancy (Kioumourtzoglou et al., 2018).
Other synthetic steroid hormones have been seen to cause brain/behavior impacts in the germline progeny in animal models. Gestational treatment with the synthetic glucocorticoid betamethasone resulted in modified brain function and behavior in guinea pigs (Moisiadis et al., 2017; Iqbal, et al., 2012). Exogenous thyroid hormone influenced brain gene expression programs and behaviors in later generations by altering germ line epigenetic information in a mouse model (Martinez et al., 2018).
It is also worth noting that in animal models, germ cell exposures to hormone-disrupting environmental chemicals have also been shown to alter brain and behavior of the offspring borne of exposed cells. For example, exposure of rats to the common fungicide vinclozolin and pollutants called PCBs at the germ cell stage led to differences in the physiological and socio-sexual phenotype in offspring, especially in males (Krishnan et al., 2018). Gestational exposure to the same compounds in rats resulted in inheritance of epigenetic errors in brain and sperm (Gillette et al., 2018). Exposure to BPA, a common plasticizer, can cause generational effects on gene expression and DNA methylation of imprinted genes in the mouse brain (Drobná et al., 2018; Wolstenholme et al., 2012).
Tobacco, tobacco components
Although it is hard to imagine now, maternal smoking was once very common. In fact it was not unusual for obstetricians of the post-war decades to prescribe smoking (and/or amphetamines) to pregnant patients as a means for weight control. This was an era in which gestating women were often instructed to not gain more than 20 pounds, and weight control measures were sometimes draconian. Unfortunately, tobacco smoke, with its hundreds of toxic chemical components, is now known to induce a wide variety of molecular aberrations in exposed tissues. This extends to germ cells.
In the first human study of its kind, grandmaternal smoking was linked to autism and autism trait risk in grandchildren through the exposed female line (Golding et al., 2017). Animal models suggest the biological plausibility of this finding. Grandpups of gestating mice exposed to nicotine exhibit hyperactivity and risk-taking behaviors (Zhu et al., 2015; Buck et al., 2019), apparently owing to alterations in gene expression in the offsprings’ brains (Buck et al., 2019).
In adult male mice, nicotine exposure also produces behavioral impairment in progeny (hyperactivity, attention deficit, and cognitive inflexibility) (McCarthy et al., 2018). Germ cell exposure to the toxic tobacco smoke component benzo[a]pyrene increases levels of germline and somatic mutation (called mosaicism) in offspring, particularly in the brain (Meier et al., 2017). The renown genetic toxicologist David DeMarini of the U.S. EPA has argued that tobacco should be considered a germ cell mutagen (DeMarini, 2012). These and other early studies are scratching the surface of the mostly unexplored realm of heritable effects of smoking, an important subject that for the first time will be the focus of a scientific workshop in Washington, DC in September 2019 (emgs-us.org).
Opiates
We are in the midst of an opioid epidemic, and, worryingly, evidence is emerging that opiates could have neurobehavioral impacts on the next generation via exposed germline (Vassoler et al., 2018; Sabzevari et al., 2018; reviewed generally in Gilardi et al., 2018).
While we have focused on brain and behavior in this discussion, other studies have demonstrated how drug, smoking or chemical exposures to germ cells can increase risks for other pathologies as well, including cancer, metabolic dysfunction and obesity, asthma and allergies, even differences in sexual behavior. Research has reported some perplexing links between autism and these conditions, and perhaps the quasi-genetic phenomenon will help explain some of them.
[Another note to biology buffs: See here for a compilation of more than 100 studies demonstrating non-genetic inheritance in humans and mammals.]
Intriguing consistencies with patterns seen in autism
It’s not every day that a hypothesis of autism comes along that can help explain many of the baffling patterns seen in the research literature. But we think this quasi-genetic hypothesis packs an unusual punch when it comes to potential explanatory power. Here are some examples:
Temporal associations. The start of the autism increase, observed to have begun with births in the 1980s, comes roughly a generation after early germ cell exposures to these novel synthetic pregnancy drugs and the peak of maternal smoking (1950s and 60s).
The 4:1 male:female sex ratio. The hypothesis is consistent with the sex-specific intergenerational responses to exposures detected in human and animal studies. Several studies in hormonal disruption of germ cells, for example, have found male offspring more likely suffer adverse effects.
Autism heterogeneity and the “broader autism phenotype.” Toxicant exposures to male or female germ cells over different times, in different doses, in different combinations, against a backdrop of varying genomic susceptibilities, would likely not cause uniform effects. This roulette-wheel mix could help explain the heterogeneity of the autisms and “broader autism phenotype” seen among other family members. Also the personalities and cognitive traits of parents themselves could have been influenced by their direct in utero exposures, as was the documented case with co-author JE who was a subject in this landmark study on developmental impacts of synthetic steroid hormone pregnancy drugs (Reinisch and Karow, 1977).
Regional, socioeconomic, and ethnic disparities. Higher rates of autism in some regions, ethnicities and socioeconomic strata may coincide with higher rates of drug exposures of the parents, for example, pregnancy smoking in the grandmother generation.
Missing heritability of autism. As discussed above, quasi-genetic effects could help explain the contrast between the strong heritability of autism and the surprisingly shallow findings from traditional DNA-sequence-focused genetics.
Arising in early brain development. It has been frequently observed that autism arises from brain mis-wiring during early development in the womb. What drives this mis-wiring? Increasingly it looks like chromatin and epigenomic factors may contribute, suggesting that “epigenetic dysfunction is a fundamental contributor to brain development and disease pathogenesis of neurodevelopmental disorders, including ASD” (Tremblay and Jiang, 2019).
Quasi-genetics as a new priority for autism research
Though we feel this hypothesis is strong, we do not remotely suggest that all autisms are quasi-genetic, or that other hypotheses are not worth exploring. Of course many are. But if we ever want to solve the mystery of autism’s heritability, research must embrace a greater degree of biological and historical authenticity. Today, we see too many researchers sitting in offices thinking in the abstract about even the weakest of genetic associations, while remaining unconcerned with any other information transmitted by germ cells, and totally disconnected from actual autism families and their complicated exposure histories. We continue to pour hundreds of millions of taxpayer dollars looking under the lamppost of gene sequencing, although it’s clear we’re in the land of diminishing returns chasing ultra-ultra rare variants with precious little relevance for families, prevention or public health. Meanwhile we spend pretty much nothing investigating heritable effects of exposures.
These questions could be researched in various ways. For example, rodent models can provide a rough idea of impacts of various drugs, such as those discussed in this article, on the next generation’s gene expression, brain function, and behavior. In humans, retrospective studies in populations with documented drug and smoking exposures could be conducted, even though of course researchers would need to be careful of “confounds,” or other factors that could be intervening to change outcomes. We suggest that the question of quasi-genetic inheritance is of such relevance and importance that our National Institutes of Health should consider funding at least 50 studies on this subject in the next three years. We dropped the ball in the 1980s when this idea first percolated. Let’s make up for all that lost time.
It is a heartbreaking possibility that errors of brain development could be an unforeseen legacy of certain benign-seeming actions that occurred very long ago. But given the potentially significant public health implications, and the emerging science demonstrating biological plausibility, it’s time to reconsider the history of our germ cells, and what those histories mean for our children.
La Donna Ford, MD is a former anesthesiologist. She is the mother of a son with idiopathic autism and lives in the San Francisco Bay Area.
Jill Escher is a research philanthropist (GermlineExposures.org), president of the National Council on Severe Autism, president of Autism Society San Francisco Bay Area, and a councilor-elect of the Environmental Mutagenesis and Genomics Society, where she also serves as chair of the Germ Cell and Heritable Effects special interest group. A former lawyer, she is the mother of two children with idiopathic autism and lives in the San Francisco Bay Area.
Correspondence may be directed to jill.escher@gmail.com.
References
Bohacek, J, Mansuy, IM. 2015. Molecular insights into transgenerational non‐genetic inheritance of acquired behaviours. Nat Rev Genet 16:641–652.
Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O'Neill HC. 2019. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacol 2019:149;66-82.
Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. 1981. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 60:794–7.
Choi CS, Gonzales EL, Kim KC, Yang SM, Kim JW, Mabunga DF, Cheong JH, Han SH, Bahn GH, Shin CY. 2016. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci Rep 6:36250.
Csoka AB, Szyf M. 2009. Epigenetic side-effects of common pharmaceuticals: A potential new field in medicine and pharmacology, Med Hypoth 73:5;770-780.
DeMarini, DM. 2012. Declaring the Existence of Human Germ-CellMutagens. Environ Mol Mutagen 53:166-172.
Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, Harris EP, Zhou C, Flaws JA, Adli M, Rissman EF. 2017. Transgenerational effects of Bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinol https://doi.org/10.1210/en.2017-00730.
Escher J. 2018. Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? Environ Epigen 4:2;dvy001.
Gilardi F, Augsburger M, Thomas A. 2018. Will Widespread Synthetic Opioid Consumption Induce Epigenetic Consequences in Future Generations? Front Pharmacol https://doi.org/10.3389/fphar.2018.00702.
Gillette R, Son MJ, Ton L, Gore AC, Crews D. 2018. Passing experiences on to future generations: endocrine disruptors and transgenerational inheritance of epimutations in brain and sperm. Epigenetics https://doi.org/10.1080/15592294.2018.1543506.
Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M.l. 2017. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 7:46179.
Iqbal K, Tran DA, Li AX, Warden C, Bai AY, Singh P, Wu X, Pfeifer GP, Szabó PE. 2015. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 16:59.
Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ. 2016. Role of histone acetylation in long-term neurobehavioral effects of neonatal exposure to sevoflurane in rats. Neurobiol Dis; 91: 209-20
Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE. 2018. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 121:2;406-4168.
Kioumourtzoglou M, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG. 2018. Association of Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental Deficits. JAMA Pediatr 172:7;670-677.
Krishnan K, Nitish Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, Gore AC. 2018. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Env Health Perspect https://doi.org/10.1289/EHP3550.
Land PC, Owen EL, Linde HW. 1981. Morphologic changes in mouse spermatozoa after exposure to inhalational anesthetics. Anesthesiology 54:53-6.
Martinez ME, Duarte CW, Stohn JP, Karaczyn A, Wu Z, DeMambro VE, Hernandez A. 2018. Thyroid hormone influences brain gene expression programs and behaviors in later generations by altering germ line epigenetic information. Mol Psychiatrydoi: 10.1038/s41380-018-0281-4. [Epub ahead of print].
McCarthy, DM, Morgan TJ, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, Bhide PG. 2018. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLOS Biol 16(10):e2006497.
Meier MJ, O'Brien JM, Beal MA, Allan B, Yauk CL, Marchetti F. 2017. In Utero Exposure to Benzo[a]Pyrene Increases Mutation Burden in the Soma and Sperm of Adult Mice. Environ Health Perspect. 125:82-88.
Moisiadis VG, Constantinof A, Kostaki A, Szyf M, Matthews SG. 2017. Prenatal Glucocorticoid Exposure Modifies Endocrine Function and Behaviour for 3 Generations Following Maternal and Paternal Transmission. Sci Rep 7:11814.
Pan JZ, Wei H, Hecker JG, Tobias JW, Eckenhoff RG, Eckenhoff MF. 2006. Rat brain DNA transcript profile of halothane and isoflurane exposure. Pharmacogenet Genomics 16:171–82.
Paul AM. 2010. Origins: How the Nine Months Before Birth Shape the Rest of Our Lives. New York: Free Press p 87.
Prokopuk, L, Hogg K, Western PS. 2018. Pharmacological inhibition of EZH2 disrupts the female germline epigenome. Clin Epigenetics 10:33.
Rampil IJ, Moller DH, Bell AH. 2006. Isoflurane modulates genomic expression in rat amygdala. Anesth Analg 102:1431–8.
Reinisch JM, Karow W. 1977. Prenatal exposure to synthetic estrogens and progestins: effects on human development. Arch Sex Behav 6:257–88.
Tang C-K, Chalon J, Markham JR, Ramanathan S.; Turndorf H. 1985. Exposure of Sires to Enflurane Affects Learning Function of Murine Progeny. Obstet Anesth Dig 5:2,67.
Titus L, Hatch EE, Drake KM, Parker SE, Hyer M, Palmer JR, Strohsnitter WC, Adam E, Herbst AL, Huo D, et al. 2019. Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): A report from the US National Cancer Institute DES Third Generation Study. Report Toxic 84:32-38.
Tremblay MW, Jiang Y-H. 2019. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu Rev Med 70:151–66.
Vutskits L, Sall JW, Jevtovic-Todorovic V. 2018. A poisoned chalice: the heritage of parental anaesthesia exposure. Brit. J. Anesth. 121;2,337-339.
Wolstenholme JT, Savera ME, Shetty RJ, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. 2012. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinol 153:3828–3838.
Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. 2014. Transgenerational Transmission of Hyperactivity in a Mouse Model of ADHD. J Neurosci 34:8;2768-2773.