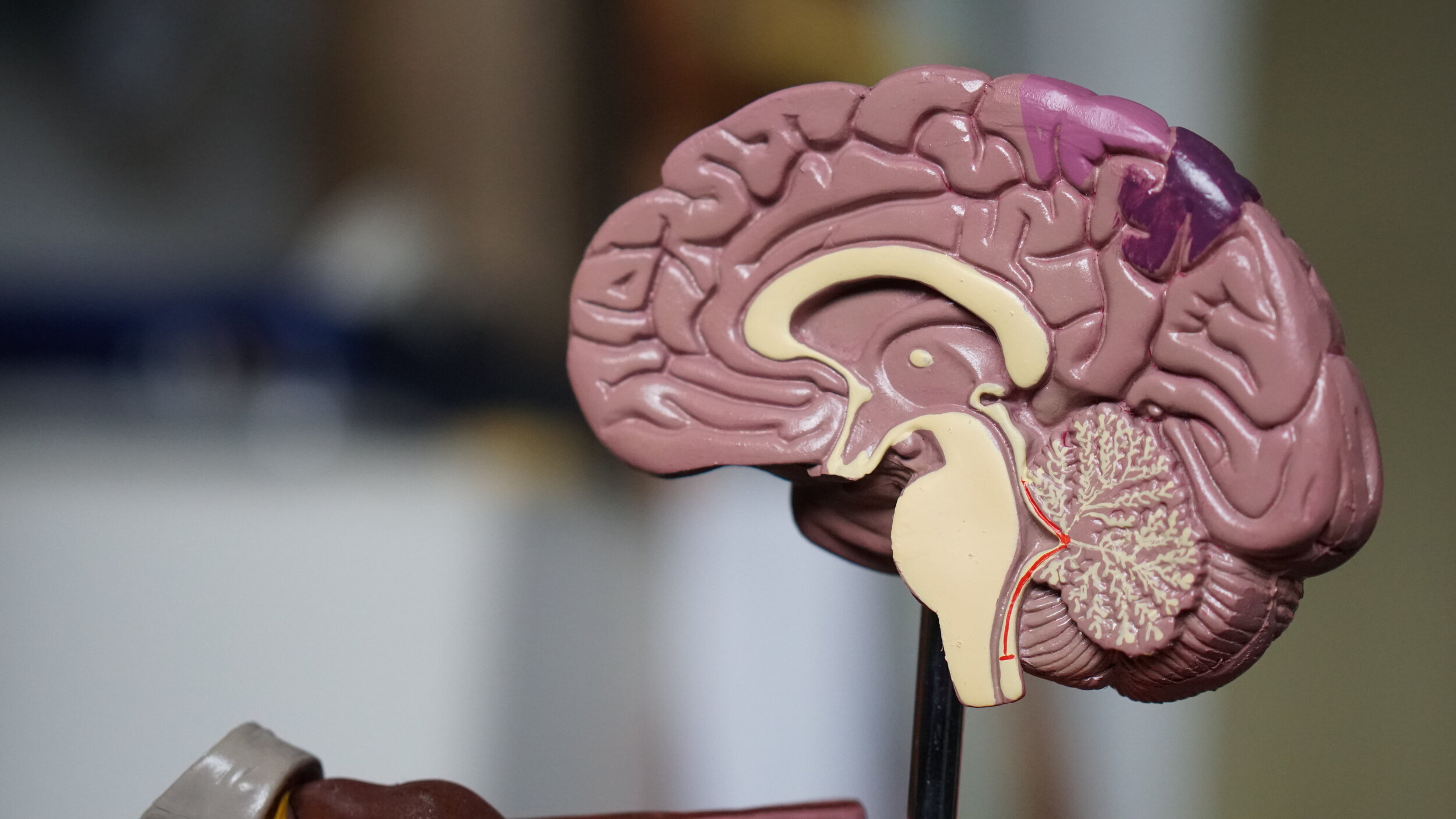
These developmental failures can have profound consequences. For example, the frontal lobe of the cortex performs complex mental functions such as reasoning and decision-making, and also contains the motor areas, which plan and execute voluntary movements. The prefrontal cortex is tied to executive functioning (the process guiding goal-directed actions and the ability to handle novel situations), attention, mental flexibility, problem-solving, verbal reasoning, working memory and the ability to switch back and forth between different tasks. When the cortical neurons are not properly connected, these all-important processes, which are deficient in autism cases, are compromised.
Another important risk is abnormal circuit function arising from improper balance of the activity between excitatory and inhibitory neurons. When projection neurons become overactive where there is not enough inhibition, epileptic seizures can result.
Of course the story does not end there. Macrocephaly, or oversized head, occurs in about 15% of autism cases, a phenomenon seen by 18 months of age, often before the onset of symptoms. Microcephaly, or abnormally small head, is seen in about 20% of cases. The big brain of autism seems to result from a pathological overabundance of neurons resulting from disturbed processes of neurogenesis in utero, followed by aberrant dendrite growth, branching, and pruning into the toddler years. Many other brain structures and processes are also implicated in autism, including the amygdala, cerebellum, corpus callosum, and left postcentral gyrus, to name some. Just as there are “many autisms” there is diversity in the brain pathology of those who exhibit autism symptoms.
But if there is a central theme from neuroscience it is that abnormal connectivity patterns — both over-connectivity and under-connectivity — compared with typically developing individuals, precedes the impairments in communication, abstract thought, sensory processing, social attention, repetitive behaviors and learning seen in autism.
BUT WHAT CAUSES BRAIN DEVELOPMENT TO GO AWRY IN AUTISM?
So what causes these mishaps in brain development? These failures do not happen out of the blue; rather, research strongly suggests they are driven by dysregulated expression of the genomic code. While some cases of autism may involve direct insults like certain in utero drug exposures like valproic acid, or perinatal complications like prematurity, by and large the neurodevelopmental pathologies of autism arise due to faulty instructions from the child’s genome. This is not to say that these cases are “genetic” in the classic sense — in fact only a small fraction fit that pattern. But how brain development and function genes are read and put to work is widely seen to deviate from the norm. Most of the autisms may not be genetic per se, but they are innate, rooted in how the genome directs the form and function of brain cells.
The genome can be thought of as an instruction book for how and when to synthesize proteins and differentiate to the end product, be it a brain cell, skin cell, or liver cell. The instruction-book has many parts. Protein-coding genes are the 1-2% of DNA that are transcribed into messenger RNA, which are then translated into polypeptide chains to make proteins. Non-coding DNA does not provide instructions for making proteins, but some of it helps control gene activity, determining when and where genes are turned on and off. Chromatin is the name for the overall physical structure of the chromosome itself, including its protein scaffolding. It helps control what the DNA does—including what DNA is accessible for protein synthesis. The epigenome includes many molecules that attach to DNA and its structure, or influence how genes are transcribed into proteins. All of these elements work together to craft the neurons and determine where and when they are born, migrate and connect up. And all of these processes have been implicated in autism.
Studies of protein-coding, or “exome,” genes have, by a wide margin, received the most attention and funding in autism research. However, this hunt for autism genes has been rather a formidable failure. No “genes for autism” have been found (see Myers et al. AHJG, 2020), and the dozens of exome genes and deletions linked with autism, are, for the most part, causative of multi-dimensional syndromes that most people do not think of as autism: for example, Fragile X, Pitt Hopkins Syndrome, Phelan-McDermid Syndrome, Angelman Syndrome, and Rett syndrome. In some instances the genes are inherited, as with Fragile X, but the majority of the time a mutation arises anew, or “de novo,” in a parent’s sperm or egg or in the conceptus, or during the earliest stages of embryonic development (called somatic mosaicism).
Most autism-linked mutations are extremely rare, occurring in far less than 1% of the autism population, and so far, taken together, these DNA sequence errors can be detected in only about 10% of cases. Nevertheless, these genes are instructive: they typically play important roles in brain development —for example, regulation of neurogenesis or synaptogenesis, whereby connections between newborn cells are formed. For example, some autism-related genetic mutations cause defects in stem cell division, leading to an overproduction of immature neurons, which alters the migration patterns of the cells and disturbs the formation of layers in the developing cortex. Others cause defects in the function of microglial cells, leading to a failure to remove unwanted synapses in the developing neural circuits.
In recent years, researchers have also discovered autism-associated mutations in non-coding sections of the genome. For example, deletions in chromosome region Xp22.11 are sometimes found in males with autism — in non-coding regions, reducing activation of the NMDA receptor, a cell-surface protein that is critical for signaling in the brain, and that plays important roles in learning and memory.
However, in the vast majority of cases, autism causes are unknown, and by my reading of the literature, finding the molecular factors disrupting the proper expression of brain development genes stands as the greatest puzzle in autism research today. Since autism is strongly heritable, the factors likely reside somewhere in the germ cells of the parents — but based on extensive evidence over two decades of research, likely not within the DNA sequence.
But wherever the faults may lie, somehow gene networks highly expressed during fetal brain development are indeed perturbed, impacting crucial signaling pathways for brain-building. This was most recently seen in a study of neurons cultured from blood cells of young children with autism, finding consistent dysregulation in a number of neural pathways. Further, the degree of dysregulation of gene expression correlated with the severity of ASD symptoms in children. Additionally, post-mortem studies have also found striking changes in gene expression in autism brains. For example, a 2016 study examining 48 post-mortem autism brains revealed that transcription levels of 584 genes were elevated in the brains of the autistic individuals compared to those of controls, whereas the levels of another 558 were reduced. Many of the dysregulated genes are expressed in microglia or neurons in the cerebral cortex. Another recent study detected almost 700 differences in gene activity in samples from 15 post mortem autism brains. The biggest differences were found in genes expressed in cells called inhibitory interneurons, which control local circuit activity, in 'projection' neurons in the upper layers of the cortex, which send fibers to neighboring regions of the brain, and in microglial cells. Dysregulation of specific sets of genes in projection neurons and microglia was associated with the severity of autism symptoms.
SUMMING UP
“In sum, while generally speaking autism is not a genetic disorder, it is a disorder of regulation of genes critical for brain development.”
In sum, while generally speaking autism is not a genetic disorder, it is a disorder of regulation of genes critical for brain development. The dysregulation disrupts successive stages of development, including cell birth, migration, dendritic growth, synapse maturation, and the assembly of circuits in the developing cerebral cortex, ultimately leading to the cognitive and behavioral dysfunctions we label as autism. Atypical gene activity is linked to the degree of autistic impairments, with those who are most impaired showing the most atypical activity.
Why does this matter? Understanding the true pathophysiology of autism should have major implications across research, medicine, and social services. For example, knowing the forces that disturb the key gene networks could lead to measures for prevention. Instead of throwing darts at costly and ineffective medical and behavioral treatments, we could more precisely and meaningfully target intervention. We could stop hitting our heads against the wall with nonsense causation theories. But most importantly, by knowing that behavior is the outgrowth of biological short-circuits (which were no one’s fault!) we could more fully understand and support all those who are disabled by autism.