Opiates
We are in the midst of an opioid epidemic, and, worryingly, evidence is emerging that opiates could have neurobehavioral impacts on the next generation via exposed germline (Vassoler et al., 2018; Sabzevari et al., 2018; reviewed generally in Gilardi et al., 2018).
While we have focused on brain and behavior in this discussion, other studies have demonstrated how drug, smoking or chemical exposures to germ cells can increase risks for other pathologies as well, including cancer, metabolic dysfunction and obesity, asthma and allergies, even differences in sexual behavior. Research has reported some perplexing links between autism and these conditions, and perhaps the quasi-genetic phenomenon will help explain some of them.
[Another note to biology buffs: See here for a compilation of more than 100 studies demonstrating non-genetic inheritance in humans and mammals.]
Intriguing consistencies with patterns seen in autism
It’s not every day that a hypothesis of autism comes along that can help explain many of the baffling patterns seen in the research literature. But we think this quasi-genetic hypothesis packs an unusual punch when it comes to potential explanatory power. Here are some examples:
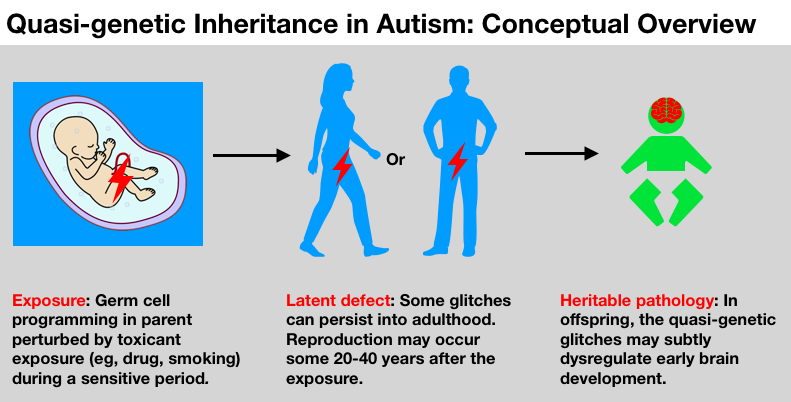
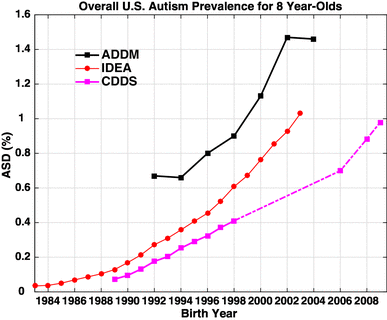
Temporal associations. The start of the autism increase, observed to have begun with births in the 1980s, comes roughly a generation after early germ cell exposures to these novel synthetic pregnancy drugs and the peak of maternal smoking (1950s and 60s).
The 4:1 male:female sex ratio. The hypothesis is consistent with the sex-specific intergenerational responses to exposures detected in human and animal studies. Several studies in hormonal disruption of germ cells, for example, have found male offspring more likely suffer adverse effects.
Autism heterogeneity and the “broader autism phenotype.” Toxicant exposures to male or female germ cells over different times, in different doses, in different combinations, against a backdrop of varying genomic susceptibilities, would likely not cause uniform effects. This roulette-wheel mix could help explain the heterogeneity of the autisms and “broader autism phenotype” seen among other family members. Also the personalities and cognitive traits of parents themselves could have been influenced by their direct in utero exposures, as was the documented case with co-author JE who was a subject in this landmark study on developmental impacts of synthetic steroid hormone pregnancy drugs (Reinisch and Karow, 1977).
Regional, socioeconomic, and ethnic disparities. Higher rates of autism in some regions, ethnicities and socioeconomic strata may coincide with higher rates of drug exposures of the parents, for example, pregnancy smoking in the grandmother generation.
Arising in early brain development. It has been frequently observed that autism arises from brain mis-wiring during early development in the womb. What drives this mis-wiring? Increasingly it looks like chromatin and epigenomic factors may contribute, suggesting that “epigenetic dysfunction is a fundamental contributor to brain development and disease pathogenesis of neurodevelopmental disorders, including ASD” (Tremblay and Jiang, 2019).
Quasi-genetics as a new priority for autism research
Though we feel this hypothesis is strong, we do not remotely suggest that all autisms are quasi-genetic, or that other hypotheses are not worth exploring. Of course many are. But if we ever want to solve the mystery of autism’s heritability, research must embrace a greater degree of biological and historical authenticity. Today, we see too many researchers sitting in offices thinking in the abstract about even the weakest of genetic associations, while remaining unconcerned with any other information transmitted by germ cells, and totally disconnected from actual autism families and their complicated exposure histories. We continue to pour hundreds of millions of taxpayer dollars looking under the lamppost of gene sequencing, although it’s clear we’re in the land of diminishing returns chasing ultra-ultra rare variants with precious little relevance for families, prevention or public health. Meanwhile we spend pretty much nothing investigating heritable effects of exposures.
These questions could be researched in various ways. For example, rodent models can provide a rough idea of impacts of various drugs, such as those discussed in this article, on the next generation’s gene expression, brain function, and behavior. In humans, retrospective studies in populations with documented drug and smoking exposures could be conducted, even though of course researchers would need to be careful of “confounds,” or other factors that could be intervening to change outcomes. We suggest that the question of quasi-genetic inheritance is of such relevance and importance that our National Institutes of Health should consider funding at least 50 studies on this subject in the next three years. We dropped the ball in the 1980s when this idea first percolated. Let’s make up for all that lost time.
It is a heartbreaking possibility that errors of brain development could be an unforeseen legacy of certain benign-seeming actions that occurred very long ago. But given the potentially significant public health implications, and the emerging science demonstrating biological plausibility, it’s time to reconsider the history of our germ cells, and what those histories mean for our children.
La Donna Ford, MD is a former anesthesiologist. She is the mother of a son with idiopathic autism and lives in the San Francisco Bay Area.
Jill Escher is a research philanthropist (GermlineExposures.org), president of the National Council on Severe Autism, president of Autism Society San Francisco Bay Area, and a councilor-elect of the Environmental Mutagenesis and Genomics Society, where she also serves as chair of the Germ Cell and Heritable Effects special interest group. A former lawyer, she is the mother of two children with idiopathic autism and lives in the San Francisco Bay Area.
Correspondence may be directed to jill.escher@gmail.com.
References
Bohacek, J, Mansuy, IM. 2015. Molecular insights into transgenerational non‐genetic inheritance of acquired behaviours. Nat Rev Genet 16:641–652.
Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O'Neill HC. 2019. Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice. Neuropharmacol 2019:149;66-82.
Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. 1981. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 60:794–7.
Choi CS, Gonzales EL, Kim KC, Yang SM, Kim JW, Mabunga DF, Cheong JH, Han SH, Bahn GH, Shin CY. 2016. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci Rep 6:36250.
Csoka AB, Szyf M. 2009. Epigenetic side-effects of common pharmaceuticals: A potential new field in medicine and pharmacology, Med Hypoth 73:5;770-780.
DeMarini, DM. 2012. Declaring the Existence of Human Germ-CellMutagens. Environ Mol Mutagen 53:166-172.
Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, Harris EP, Zhou C, Flaws JA, Adli M, Rissman EF. 2017. Transgenerational effects of Bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinol https://doi.org/10.1210/en.2017-00730.
Escher J. 2018. Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? Environ Epigen 4:2;dvy001.
Gilardi F, Augsburger M, Thomas A. 2018. Will Widespread Synthetic Opioid Consumption Induce Epigenetic Consequences in Future Generations? Front Pharmacol https://doi.org/10.3389/fphar.2018.00702.
Gillette R, Son MJ, Ton L, Gore AC, Crews D. 2018. Passing experiences on to future generations: endocrine disruptors and transgenerational inheritance of epimutations in brain and sperm. Epigenetics https://doi.org/10.1080/15592294.2018.1543506.
Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M.l. 2017. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep 7:46179.
Iqbal K, Tran DA, Li AX, Warden C, Bai AY, Singh P, Wu X, Pfeifer GP, Szabó PE. 2015. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 16:59.
Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ. 2016. Role of histone acetylation in long-term neurobehavioral effects of neonatal exposure to sevoflurane in rats. Neurobiol Dis; 91: 209-20
Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE. 2018. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Brit J Anesth 121:2;406-4168.
Kioumourtzoglou M, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG. 2018. Association of Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental Deficits. JAMA Pediatr 172:7;670-677.
Krishnan K, Nitish Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, Gore AC. 2018. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Env Health Perspect https://doi.org/10.1289/EHP3550.
Land PC, Owen EL, Linde HW. 1981. Morphologic changes in mouse spermatozoa after exposure to inhalational anesthetics. Anesthesiology 54:53-6.
Martinez ME, Duarte CW, Stohn JP, Karaczyn A, Wu Z, DeMambro VE, Hernandez A. 2018. Thyroid hormone influences brain gene expression programs and behaviors in later generations by altering germ line epigenetic information. Mol Psychiatrydoi: 10.1038/s41380-018-0281-4. [Epub ahead of print].
McCarthy, DM, Morgan TJ, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, Bhide PG. 2018. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLOS Biol 16(10):e2006497.
Meier MJ, O'Brien JM, Beal MA, Allan B, Yauk CL, Marchetti F. 2017. In Utero Exposure to Benzo[a]Pyrene Increases Mutation Burden in the Soma and Sperm of Adult Mice. Environ Health Perspect. 125:82-88.
Moisiadis VG, Constantinof A, Kostaki A, Szyf M, Matthews SG. 2017. Prenatal Glucocorticoid Exposure Modifies Endocrine Function and Behaviour for 3 Generations Following Maternal and Paternal Transmission. Sci Rep 7:11814.
Pan JZ, Wei H, Hecker JG, Tobias JW, Eckenhoff RG, Eckenhoff MF. 2006. Rat brain DNA transcript profile of halothane and isoflurane exposure. Pharmacogenet Genomics 16:171–82.
Paul AM. 2010. Origins: How the Nine Months Before Birth Shape the Rest of Our Lives. New York: Free Press p 87.
Prokopuk, L, Hogg K, Western PS. 2018. Pharmacological inhibition of EZH2 disrupts the female germline epigenome. Clin Epigenetics 10:33.
Rampil IJ, Moller DH, Bell AH. 2006. Isoflurane modulates genomic expression in rat amygdala. Anesth Analg 102:1431–8.
Reinisch JM, Karow W. 1977. Prenatal exposure to synthetic estrogens and progestins: effects on human development. Arch Sex Behav 6:257–88.
Tang C-K, Chalon J, Markham JR, Ramanathan S.; Turndorf H. 1985. Exposure of Sires to Enflurane Affects Learning Function of Murine Progeny. Obstet Anesth Dig 5:2,67.
Titus L, Hatch EE, Drake KM, Parker SE, Hyer M, Palmer JR, Strohsnitter WC, Adam E, Herbst AL, Huo D, et al. 2019. Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): A report from the US National Cancer Institute DES Third Generation Study. Report Toxic 84:32-38.
Tremblay MW, Jiang Y-H. 2019. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu Rev Med 70:151–66.
Vutskits L, Sall JW, Jevtovic-Todorovic V. 2018. A poisoned chalice: the heritage of parental anaesthesia exposure. Brit. J. Anesth. 121;2,337-339.
Wolstenholme JT, Savera ME, Shetty RJ, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. 2012. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinol 153:3828–3838.
Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. 2014. Transgenerational Transmission of Hyperactivity in a Mouse Model of ADHD. J Neurosci 34:8;2768-2773.